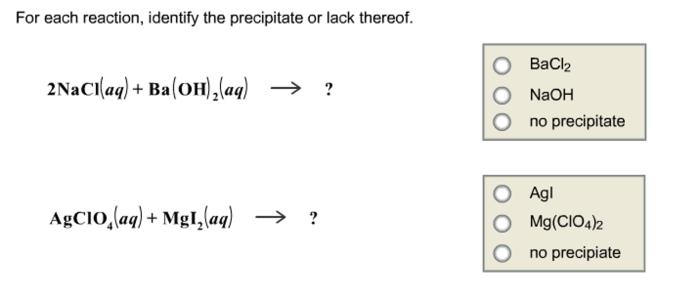
Identify the precipitate or lack thereof for the reaction – Identifying precipitates and understanding the reasons for their absence is a crucial aspect of chemistry. This comprehensive guide delves into the intricacies of precipitation reactions, providing a clear understanding of the factors that govern precipitate formation and their applications in various scientific disciplines.
Precipitation reactions are chemical reactions that result in the formation of an insoluble solid compound, known as a precipitate. These reactions play a significant role in analytical chemistry, environmental science, and various industrial processes.
Precipitation Reaction Fundamentals: Identify The Precipitate Or Lack Thereof For The Reaction
Precipitation reactions occur when two soluble ionic compounds in an aqueous solution react to form an insoluble solid compound, called a precipitate. These reactions are often used in qualitative analysis to identify ions in solution.
Examples of precipitation reactions include:
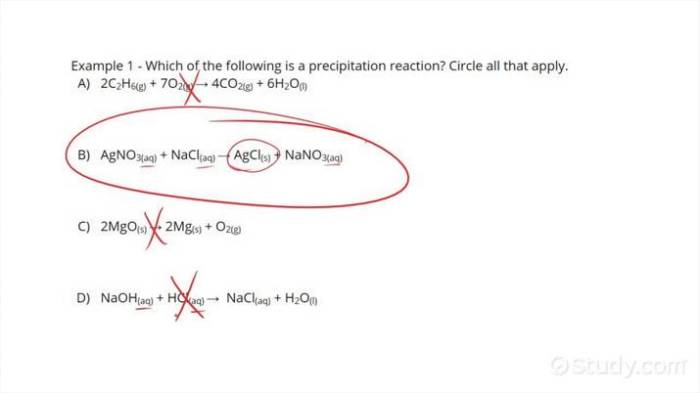
- AgNO 3(aq) + NaCl(aq) → AgCl(s) + NaNO 3(aq)
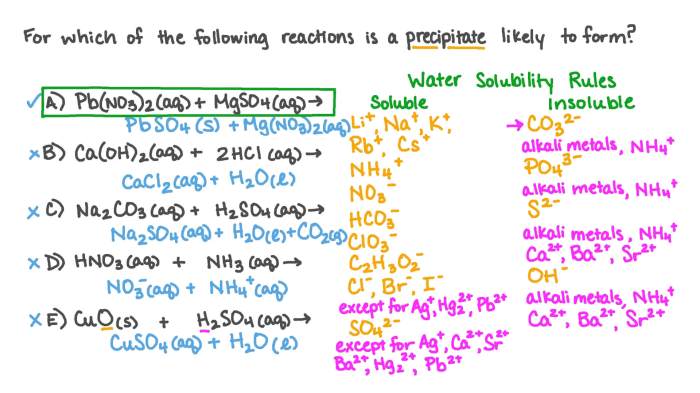
- Pb(NO 3) 2(aq) + Na 2SO 4(aq) → PbSO 4(s) + 2NaNO 3(aq)
- BaCl 2(aq) + Na 2SO 4(aq) → BaSO 4(s) + 2NaCl(aq)
Factors that influence precipitation reactions include:
- Concentration of the reactants
- Temperature
- pH
Identifying Precipitates
Precipitates are typically characterized by their:
- Solid form
- Low solubility in the solvent
- Formation of a cloudy or opaque solution
Precipitates can be identified using various methods, including:
- Appearance
- Solubility
- Use of solubility rules
Solubility rules are a set of guidelines that can be used to predict whether a precipitate will form in a given reaction. For example, according to the solubility rules, all alkali metal salts (e.g., Na +, K +), ammonium salts (NH 4+), nitrate salts (NO 3–), and chloride salts (Cl –) are soluble in water.
Therefore, reactions involving these ions will not typically form precipitates.
Absence of Precipitates
Some reactions do not form precipitates because the products are soluble in the solvent. This can occur when:
- The products are both strong electrolytes
- The products form a complex ion
For example, the reaction between sodium chloride (NaCl) and potassium chloride (KCl) does not form a precipitate because both products are soluble in water. Similarly, the reaction between copper(II) sulfate (CuSO 4) and sodium hydroxide (NaOH) does not form a precipitate because the products form a complex ion, [Cu(OH) 4] 2-.
Applications of Precipitate Identification, Identify the precipitate or lack thereof for the reaction
Precipitate identification is important in various fields, including:
- Analytical chemistry: Precipitates can be used to separate and identify ions in solution.
- Environmental science: Precipitates can be used to remove pollutants from water and wastewater.
- Pharmaceutical industry: Precipitates can be used to produce drugs and other pharmaceutical products.
Real-world examples of precipitate identification applications include:
- The use of silver nitrate to test for the presence of chloride ions in water
- The use of barium chloride to test for the presence of sulfate ions in water
- The use of lead acetate to test for the presence of sulfide ions in water
FAQ Insights
What are the key characteristics of precipitates?
Precipitates are typically solids that form when two solutions are mixed, resulting in the formation of an insoluble compound. They often appear cloudy or opaque and can vary in color and texture.
How can we predict whether a reaction will form a precipitate?
Solubility rules provide a useful tool for predicting precipitate formation. These rules Artikel the solubility of various ions in water, allowing us to determine whether a reaction will result in a precipitate or not.
What factors can prevent the formation of precipitates?
Factors such as high solubility, the formation of complex ions, and the presence of certain solvents can prevent the formation of precipitates, even in reactions where insoluble compounds are expected.